There are plenty of chemicals in a laboratory that are harmless. There are also plenty of chemicals which are highly dangerous for your health. These chemicals are labelled with a pictogram which defines what type of hazard you may encounter. Learn to recognize the following pictograms.
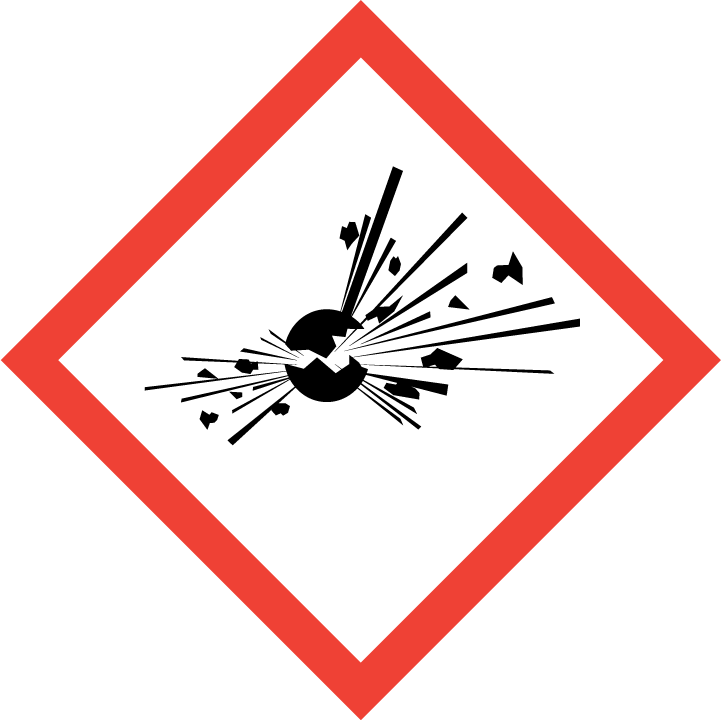
Explosive
Explosives
Self-Reactives
Organic Peroxides
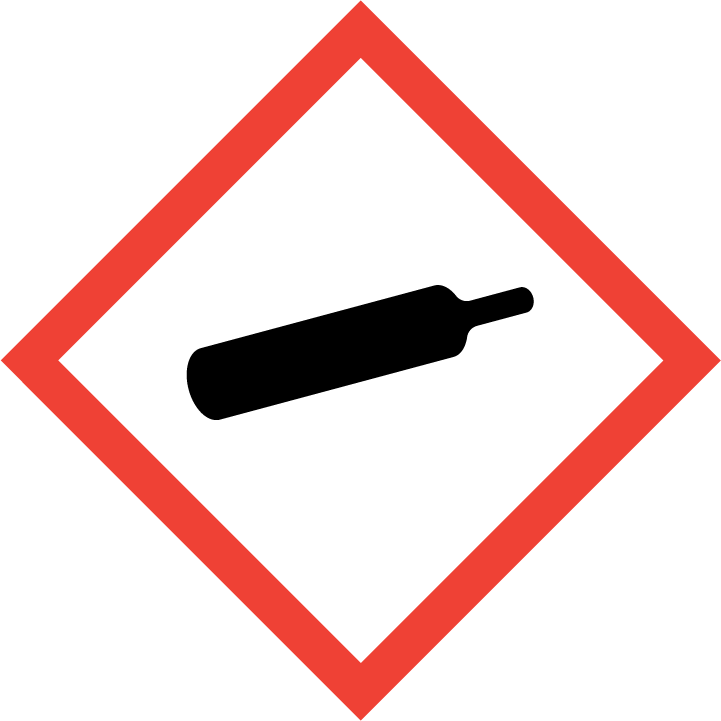
Compressed gas
Gases Under Pressure

Harmful
Irritant (skin and eye)
Skin Sensitizer
Acute Toxicity (harmful)
Narcotic Effects
Respiratory Tract Irritant
Hazardous to Ozone Layer (Non-Mandatory)

Flammable
Flammables
Pyrophorics
Self-Heating
Emits Flammable Gas
Self-Reactives
Organic Peroxides
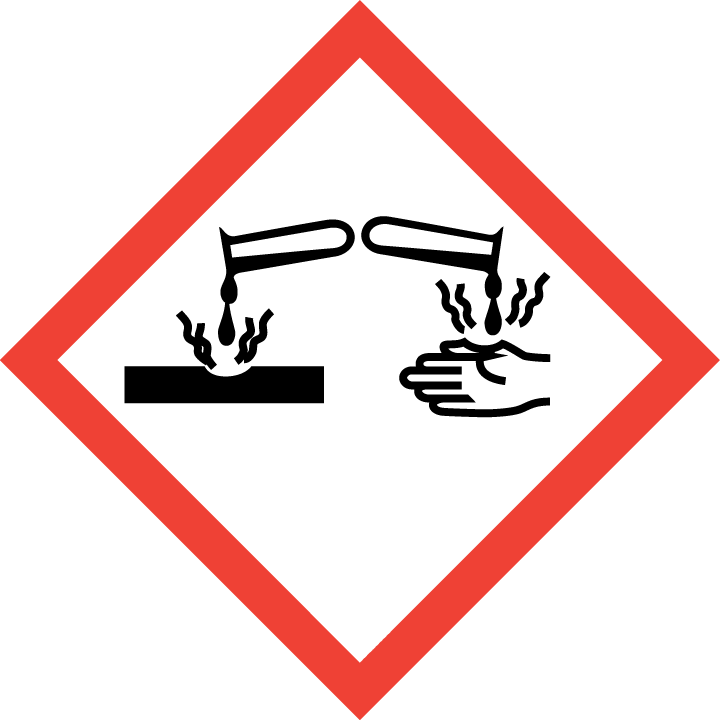
Corrosion
Skin Corrosion/Burns
Eye Damage
Corrosive to Metals
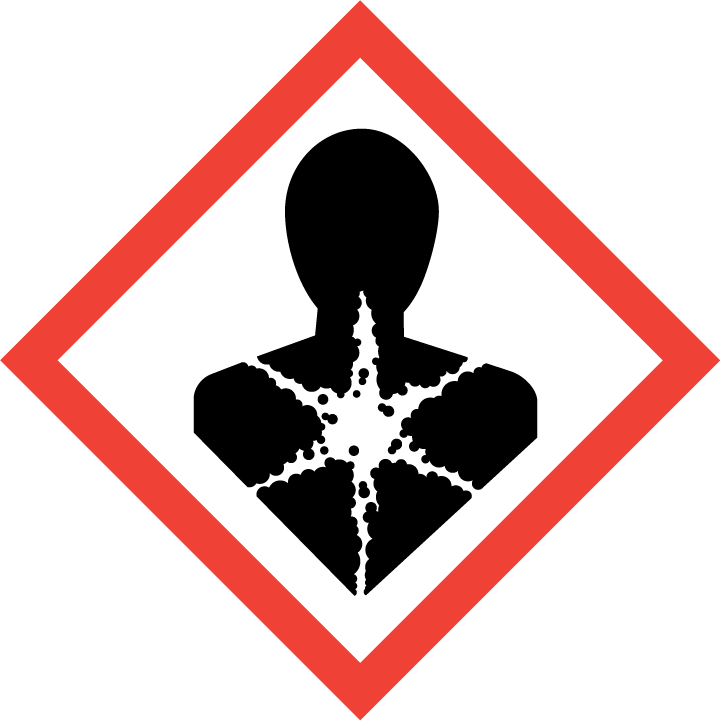
Health hazard
Carcinogen
Mutagenicity
Reproductive Toxicity
Respiratory Sensitizer
Target Organ Toxicity
Aspiration Toxicity
Oxidizing
Oxidizers

Toxic
Acute Toxicity (fatal or toxic)
Environment
Aquatic Toxicity
It is important that you learn and understand the meaning of these symbols as they tell you what the risks are, what to think of when working with the corresponding chemicals, which safety equipment to consider when manipulating them (gloves, safety goggles, ventilated hood, etc.).
Additionally, you will find safety statements and codes which define more precisely the danger encountered when manipulating specific chemicals. They are:
- Hazard statements or H-statements (“H” followed by a number, which describe the nature of the risks of a substance or mixture),
- Precautionary statements or P-statements (“P” followed by a number, which indicate how hazards may be prevented or reduced).
Check the following page for more info: Chemical inventory, exposure register and safety labeling | The HSE-gateway | UiB
In the following example, the hazardous substance is clearly labeled with pictograms, H- and P-statements.
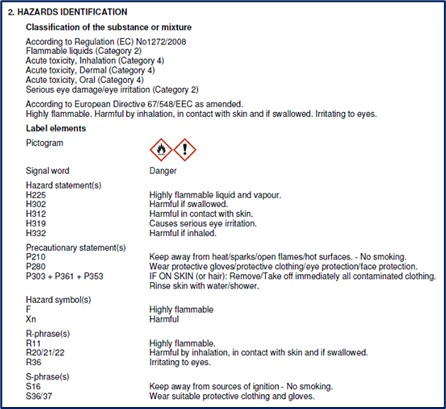
Chemical register & Exposure register
All chemicals are delivered with a Safety Data Sheet (SDS or MSDS), which describes in details the substance or mixture, the risks and dangers, the actions to take in case of exposure, fire, waste and disposal. You must always read datasheets before starting a procedure which involves hazardous chemicals. Use this link to open the chemical inventory (stoffkartoteket), which gives access to all datasheets of all chemicals stored or in use at your workplace. Log in with Feide to get access to the chemical inventory system. If it appears to be difficult to find the SDS in English via the chemical inventory system or any other online source (manufacturer), contact either your lab manager or the HSE coordinator at BIO.
Exposure register is a personal register where you can log or report single exposures as well as repeated exposures to carcinogenic, mutagenic or reproductively harmful chemicals (Carc. 1A, Carc. 1B, Muta. 1A or Muta. 1B ), i.e. substances marked with the H-statements H340, H350, H350i and all H360-statements. If you use, have used or plan to use such chemicals, you shall carry out the registration yourself. If you have questions contact your lab-manager or the HSE coordinator at BIO. More information regarding registration of exposures is available on the HSE-gateway. Chemical inventory, exposure register and safety labeling | The HSE-gateway | UiB.
Precautions when working with dry ice
Dry ice is CO₂ in solid form. It sublimates, i.e. it evaporates directly into CO₂ gas that displaces air and can cause suffocation. Dry ice has a temperature of -78°C and can cause severe frost damage upon skin contact. Wear protective gloves when handling dry ice.
At 15°C, 1kg of dry ice will expand to 530L when it sublimates to gas form. CO₂ gas is 1.5 times heavier than air, is colorless and odorless. A single sublimation test showed that 25-30% of the dry ice sublimed at minus 20 degrees within 1 day. At room temperature 50-60% of the dry ice sublimed within 1 day.
Symptoms of inhalation of CO₂-containing air
2-4%: Increased breathing activity and headache.
4-6%: Headache, feeling sick and vomiting. May cause unconsciousness if the affected person does not quickly get into fresh air.
10%: Circulatory disturbances leading to coma and death. Good ventilation is therefore important in handling and storing dry ice.
Storage of dry ice
The symbol to the right is found on containers or freezers used to store dry ice (full sign available here).
Minus 80 degrees freezers (ultra freezers) are best suited for storing dry ice. Dry ice will over time sublimate in a BIO freezer as well, the volume will increase and cause increased pressure if the freezer is tightly closed. In freezers, CO₂ will lie in the bottom and be able to build up to dangerous concentration. Do not put your head down in a freezer that contains dry ice.

Dry ice can be stored for a shorter period in the Backup freezers in Room 2B07, 2nd floor A-block and room 526B1, 5th floor BIO-block, and room 432B2, 4th floor BIO-block. These freezers must be labeled properly.
If it is necessary to store larger amounts of dry ice, other ultra-freezers can be used providing that the BIO Emergency Guard (453 92 771) is informed and approves it, and that the freezer is marked properly. Larger amounts of dry ice should not be stored for a long time.
Precautions when transporting and handling liquid nitrogen
Liquid nitrogen has a temperature of -196 °C and can cause severe frostbite on skin contact. When nitrogen evaporates in tight spaces (elevators, rooms with minimal ventilation) a lack of oxygen can occur. 1 liter of liquid nitrogen expands to 706L when it changes to gaseous form and displaces air. It is therefore important to take precautions when transporting and using liquid nitrogen.
Transportation
The container with liquid nitrogen must always be sent alone in the lift due to the risk of suffocation that may arise. The container must be marked with the sign "LIQUID NITROGEN TRAVELS ALONE IN THE ELEVATOR - UNIVERSITY OF BERGEN Department of Biological Sciences AKF, version 2.1 2 23/06/2023 . There should be two people to pick up a container filled with liquid nitrogen so that the container can be sent alone in a lift with one person downstairs and one upstairs. Wait for the elevator to return empty. If containers are to be transported outside the house, they must be secured with straps on the wheel set and the wheels must be lockable.

Protective equipment
- Wear a laboratory coat, possibly a leather apron, a face shield, gloves that protect against the cold and tight footwear with the trousers pulled over the shoes.
- Avoid rolled up trousers where liquid nitrogen can collect and cause frostbite.
First aid measures
If you get liquid nitrogen on bare skin, you should not rub the wound, but use lukewarm water for at least 15 minutes. Always consult a doctor for deeper frostbite or tissue damage.
More information in this document.

